
Written by: Becky Anderson
Published by: David Santos
Ralstonia solanacearum
What is Ralstonia solanacearum?
The bacterial pathogen Ralstonia solanacearum is a Gram-negative, soil-borne bacterial pathogen that causes bacterial wilt and brown rot in potatoes. This bacterium belongs to a complex of four groups called phylotypes. These groups are known as the R. solanacearum species complex (RSSC). Each group is dominant in a geographical region (Table 1). Bacterial wilt caused by R. solanacearum targets the Solanaceae family of plants, including eggplants, tomatoes, potatoes, tobacco and peppers.
Phylotype – a taxonomic grouping of bacteria based on how similar their genes are
Table 1. Phylotypes of the Ralstonia solanacearum species complex and their region of predominance.
|
Group/Phylotype |
Predominant Region* |
|
I |
Asia |
|
II |
America |
|
III |
Africa |
|
IV |
Indonesia, Japan, the Philippines and Australia |
*Information summarized from Guidot et al. 2007 and Safni et al. 2014 [1;2].
The yield loss of Solanaceae crops caused by R. solanacearum is estimated to range from 15-55% globally [3]. However, the yield loss can be even higher, as observed in Uganda, where a farm experienced an 88% yield loss of their tomato crops, and a farm in Ethiopia experienced a 100% loss of their pepper plants, both situations caused by R. solanacearum [4]. It is no surprise that bacterial wilt caused by R. solanacearum has economic repercussions. It is estimated that 80 counties with over 1.7 million hectares of potato plants are affected annually, resulting in a $950 million loss annually [5,6].
These numbers don’t take into account the effect on hydroponic farms. However, you can imagine the devastation a hydroponic system would face if it were infected with R. solanacearum. The good news is there are methods to treat your crops to prevent bacterial wilt; keep reading to find out what they are (spoiler alert, Healthy Hydroponics screens for R. solanacearum to provide early detection through routine surveillance)!
Infection and Symptoms:
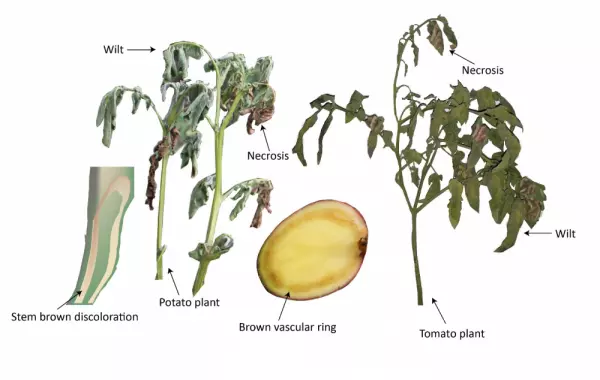
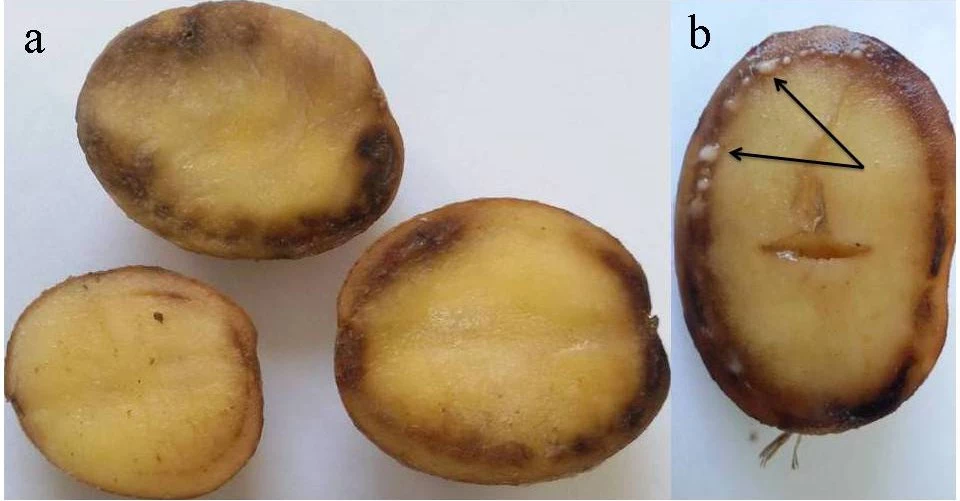
Characteristic symptoms of brown rot and bacterial wilt include yellowing or browning, wilting and necrosis of the leaves, and browning or discoloration of the vascular tissue (Figure 1). Specifically in potatoes, when cut at a cross-section, you will see a brown ring just under the skin (Figures 1 and 2) and bacterial secretion from the vascular ring (Figure 2). Once R. solanacearum has infected and replicated in the plant, it becomes motile and can be isolated from any part of the plant [5].
Ralstonia solanacearum enters the roots through broken tissue or natural openings and proliferates in the vascular tissues. This results in interrupted xylem function and can ultimately result in the death of the plant [5]. Lipopolysaccharides are produced by R. solanacearum, which blocks the xylem and prevents water from reaching the leaves, resulting in wilting [7].
Vascular tissues – an arrangement of cells in a plant, comprised of the xylem and phloem, which provide transport of water, sugars and nutrients, structural support
Xylem – plant vascular tissue that transports water and minerals from the roots to the rest of the plant, also provides structural support
Lipopolysaccharide – a molecule that is a major component in the cell wall of some bacteria (Gram-negative bacteria)
Necrosis – the death of cells or tissue
We provide great overviews of many agricultural microorganisms. Subscribe to stay updated!
Growth Conditions and Lifecycle:
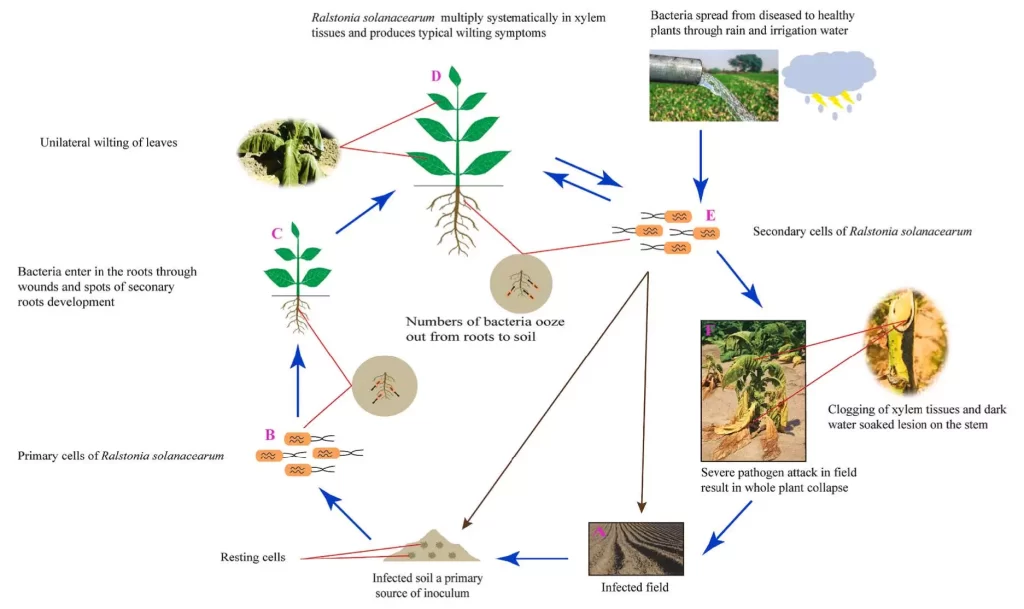
Ralstonia solanacearum is soilborne, but they can also inhabit water. This bacterium can reside in soil or water for up to 40 years without a host plant [5]. However, the viability of this pathogen is lost under extreme conditions such as high pH, temperature and salinity [5]. Once R. solanacearum has completed its life cycle (Figure 3), bacterial cells ooze out from the rhizosphere, which then enters and proliferates in the soil. Or, in the case of hydroponic systems, the bacterial cells will enter the irrigation water, which feeds into the rest of the crop.
Viability – the ability to survive and function successfully
Pathogen – a virus, bacteria or other microorganisms that can cause disease
Rhizosphere – the region surrounding the plant’s roots, which is comprised of microorganisms that are directly influenced by the plant’s health and functionality
Methods of control:
Ralstonia solanacearum can spread through water or on contaminated surfaces, including seeds and equipment. Therefore, once an infection is identified, diseased plants should be quarantined and treated appropriately. Additionally, water supplies and equipment need to be treated and disinfected. Currently, there are a few different approaches to treat or prevent disease caused by R. solanacearum, including chemical treatments, biocontrol methods, or incorporating resistant cultivars.
Chemical Treatments:
There are many types of chemical treatments on the market to target various pathogens. These chemical treatments include chlorine dioxide, which can be used as a disinfectant in water or on contaminated surfaces, such as equipment [8].
A peroxyacetic acid mixture [9] or salicylic acid and potassium silicate [10] can also be used.
Biocontrol Methods:
If you are looking for a more natural approach to control pathogens in your crops, biocontrol may be the best choice for you (be sure to check out our blog on biocontrol methods). Various bacterial species can be used for biocontrol; two categories of these bacteria include endophytes and rhizobacteria. These bacterial species include the endophytic Enterobacter cloacae and the rhizobacterial Paenibacillus polymyxa [5]. Both bacteria suppressed the incidence of bacterial wilt in potatoes by 26.5% and 80%, respectively [5].
Biocontrol – the use of living organisms or natural resources to reduce or prevent damage caused by dangerous organisms
Endophyte – an organism, usually a bacteria or fungus, that lives inside a plant
Rhizobacteria – bacteria that reside in and around the roots of plants which can have a beneficial, neutral or detrimental effect on the plant’s health and growth
Additionally, bacteriophages, also known as phages, can be used to control diseases caused by bacteria [11]. Phages are viruses that target specific bacteria by attaching to their cell wall, injecting their DNA or RNA, replicating inside the bacteria, and lysing the bacterial cell to disseminate more phage particles.
Bacteriophage – a virus that targets and infects bacteria, replicates inside it and can ultimately kill the bacteria
Resistant Cultivars:
If you want to implement proactive measures right off the bat or want to try a natural and organic solution to your bacterial wilt problems, incorporating some resistant cultivars may work as a solution. Studies have shown that some cultivars of plants are naturally resistant to R. solanacearum infection. In tomato plants, cultivar Hawaii7996 showed resistance to bacterial wilt caused by R. solanacearum (Figure 4) [12]. Other resistant cultivars include wild tomatoes, Solanum lycopersicum and S. peruvianum [13]. Research is ongoing, trying to modify some plant cultivars to incur genetic resistance. However, due to the diverse conditions in which plants grow (temperature, humidity, pH, salinity and nutrients), it is challenging to develop a resistant plant that is stable in all conditions.

Overall, bacterial wilt or brown rot caused by R. solanacearum is an economically, mentally, and emotionally draining issue. The treatment methods mentioned above are mostly reactive measures instead of proactive precautions—Healthy Hydroponics screens for R. solanacearum and many other pathogens of concern in your hydroponic system. You will be notified when pathogens of concern show up in your system before their bacterial load gets too high to treat. Feel free to send us an email to learn more at info@healthyhydroponics.ca. Your hydroponic systems will thank you!
Disclaimer:
The information we present in Pathogen Profile is based on collating published peer-reviewed scientific literature and sources we think are reliable. This is by no means an exhaustive review of pathogens. Pathogen Profile gives a small glimpse of what is known about pathogens and we encourage growers to do more research on their own based on the pathogens in relation to their own crops and hydroponic systems. We are not plant pathologists thus the information presented in Pathogen Profile should not be used as professional advice to treat pathogens or operate your hydroponic system.
References
- Guidot, A. et al. Genomic structure and phylogeny of the plant pathogen ralstonia solanacearum inferred from gene distribution analysis. J Bacteriol 189, 377–387 (2007)
- Safni I, Cleenwerck I, DeVos P, Fegan M, Sly L, Kappler U. Polyphasic taxo- nomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solan- acearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol. 2014;64:3087–103.
- Kim, B.-S., French, E., Caldwell, D., Harrington, E.J., Iyer-Pascuzzi, A.S., 2016. Bacterial wilt disease: host resistance and pathogen virulence mechanisms. Physiol. Mol. Plant. Pathol. 95, 37–43. https://doi.org/10.1016/j.pmpp.2016.02.007.
- Mamphogoro, T.P., Babalola, O.O., Aiyegoro, O.A., 2020. Sustainable management strategies for bacterial wilt of sweet peppers (Capsicum annuum) and other Solanaceous crops. J. Appl. Microbiol. https://doi.org/10.1111/jam.14653.
- Ahmed, W. et al. Ralstonia solanacearum, a deadly pathogen: Revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 21, 100479 (2022).
- Charkowski, A., Sharma, K., Parker, M. L., Secor, G. A. & Elphinstone, J. Bacterial Diseases of Potato. in The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind (eds. Campos, H. & Ortiz, O.) 351–388 (Springer International Publishing, 2020). doi:10.1007/978-3-030-28683-5_10.
- Genin S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010;187:920–8.
- Popović, T., Ivanović, Ž., Janjatović, S., Ignjatov, M. & Milovanović, P. Chlorine dioxide as a disinfectant for Ralstonia solanacearum control in water, storage and equipment. Ratarstvo i povrtarstvo (2016).
- Hong JK, Jang SJ, Lee YH, Jo YS, Yun JG, Jo H, Park CJ, Kim HJ. Reduced Bacterial Wilt in Tomato Plants by Bactericidal Peroxyacetic Acid Mixture Treatment. Plant Pathol J. 2018 Feb;34(1):78-84. doi: 10.5423/PPJ.NT.06.2017.0131. Epub 2018 Feb 1. PMID: 29422791; PMCID: PMC5796753.
- Jiang, N.-H. & Zhang, S.-H. Effects of Combined Application of Potassium Silicate and Salicylic Acid on the Defense Response of Hydroponically Grown Tomato Plants to Ralstonia solanacearum Infection. Sustainability 13, 3750 (2021).
- Álvarez B, López MM and Biosca EG (2019) Biocontrol of the Major Plant Pathogen Ralstonia solanacearum in Irrigation Water and Host Plants by Novel Waterborne Lytic Bacteriophages. Front. Microbiol. 10:2813. doi: 10.3389/fmicb.2019.02813
- 이지현, 장경수, 최용호, 김진철 & 최경자. Development of an Efficient Screening System for Resistance of Tomato Cultivars to Ralstonia solanacearum. Res. Plant Dis 21, 290–296 (2015).
- Kim, S. G. et al. Evaluation of Resistance to Ralstonia solanacearum in Tomato Genetic Resources at Seedling Stage. The Plant Pathology Journal 32, 58–64 (2016).




